Soil Chemistry : pH
The acidity or basicity of a soil is measured using pH – a negative logarithmic scale of the activity of hydrogen ions in a solution. The amount of acidity depends on factors such as the mineral composition, rainfall, microbial populations, plant growth and respiration, as well as anthropogenic influences like air pollution and fertilizer use.
Soil processes including nutrient availability, biological activity, structural stability and biogeochemical cycling are significantly influenced by soil pH and we are likely to find different plants, fungi and invertebrates in acid or alkaline soils. By monitoring the soil pH and looking at variation across the OpenLiving Lab site, we can learn more about how the soils have been influenced by the topography, human influences and management, and see how this relates to other biogeochemical factors and biodiversity.

Methods of measuring pH
We collected soil samples from the riparian woodland in 2023, the urban woodland in 2024 and the floodplain meadow in 2025 from the same sites as the cores were taken to assess soil compaction. Samples were collected from the topsoil (0-10 cm depth) and subsoil (10-20 cm depth) using an auger.
Once the soils have been collected the samples are processed to remove any roots and stones and chopped finely before drying at 40 °C for up to 7 days. The dried soils are then ground in a pestle and mortar and passed through a sieve to make sure all soil particles are < 2 mm.
To measure the pH we need to make a soil suspension with a soil:water ratio of 1:5. To do this, 5 g of soil from each sample is weighed out and suspended in 25 ml distilled water in 50 mL centrifuge tube, which is then shaken at 120 rpm for an hour. The solution is then measured using a digital pH meter. Every 10th sample is weighed out and measured three times to check for reproducibility.
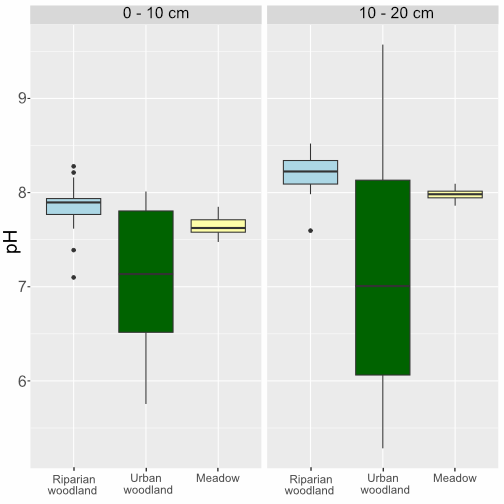
OpenLiving Lab soil pH results
Soils tested in the OpenLiving Lab ranged from pH 5.28 to pH 9.57, with most being neutral-alkaline (around pH 7.5-8). The deeper soils from 10-20cm depth were more alkaline (around pH 8), but the surface topsoils in the floodplain meadow and riparian woodland were more neutral, with a mean pH of 7.4 .
| Depth | Area | mean pH | st. dev. pH |
| Topsoil (0-10cm) | Riparian | 7.43 | 0.6 |
| Topsoil (0-10cm) | Urban woodland | 7.8 | 0.19 |
| Topsoil (0-10cm) | Meadow | 7.4 | 0.58 |
| Subsoil (10-20cm) | Riparian | 7.67 | 1.04 |
| Subsoil (10-20cm) | Urban woodland | 8.11 | 0.16 |
| Subsoil (10-20cm) | Meadow | 7.46 | 0.92 |
Urbanisation effects on soil pH
While pollution is usually linked to acidification of natural soils, in an urban context the picture is more complex. Urbanisation has actually been linked to forest soils becoming more alkaline, possibly due to the release of calcium from building rubble and weathering of concrete structures, as well as irrigation, and salt used for de-icing roads and pavements.
Related OU Research
Holly Woo has been analysing the pH of ancient woodland soils in Milton Keynes to see if urbanisation or visitor usage is affecting soil chemistry in these semi-natural habitats. She has been comparing the pH of soil samples taken from Shenley Wood to the Ellenberg values of plants found near the sampling locations. She can see where in the wood the soils are more acidic or alkaline and see if plants adapted to different conditions are growing in these localities.
References / Further reading
Soil sampling and methods of analysis (second edition). 2006. Edited by M.R. Carter & E.G. Gregorich. CRC Press, Boca Raton, Florida, USA.
Zhang et al., 2023. Urban forest soil is becoming alkaline under rapid urbanization: A case study of Changchun, northeast China. CATENA 224:106993.
Craul., P. 1985. A Description Of Urban Soils And Their Desired Characteristics. Arboriculture & Urban Forestry 11:330-339.

